Abstract
Background: Clinical trials of axicabtagene ciloleucel (axi-cel) have demonstrated favorable efficacy compared to conventional salvage therapy for the treatment of r/r LBCL (Neelapu et al. Blood Adv 2021; Locke et al. NEJM 2022). In the third or later line settings, a favorable overall response rate (ORR) for axi-cel was observed among patients (pts) aged ≥ 65 years compared with younger pts in a Center for International Blood and Marrow Transplant Research (CIBMTR) analysis (Jacobson et al. Transplant Cell Ther 2022). Here we compared, for the first time, effectiveness outcomes between treatment with either axi-cel or chemoimmunotherapy (CIT) in an elderly population in real-world settings.
Methods: From 76 US centers, a total of 1146 pts treated with commercial axi-cel for r/r LBCL in the third or later line settings between October 2017 and August 2020 were identified from a non-interventional post-authorization safety study using the CIBMTR registry. A separate cohort of 469 pts treated with CIT for r/r LBCL after ≥ 2 lines of prior therapy between 2001 and 2014 were identified from the SCHOLAR-1 data set (Crump et al. Blood 2017). Pts with the following criteria were excluded: a disease histology other than LBCL, unknown or < 2 prior lines of therapy, or prior allogeneic stem cell transplant. Two analysis sets were created: a Response Rate Analysis Set, which consisted of pts whose ORR and complete response (CR) data were available, and a Survival Analysis Set, which included pts for whom overall survival (OS) data were available. Propensity score (PS) matching was performed separately for the two analysis sets to balance distribution of baseline characteristics between axi-cel and CIT groups. Proportions and 95% Clopper-Pearson confidence intervals (CIs) for ORR and CR, and Kaplan-Meier estimates for OS, were calculated in the PS-matched sets. Multivariable logistic and Cox regressions were also conducted to further adjust residual confounding effects after PS matching. Adjusted curves were generated based on the direct adjusted survival function (Makuch J Chronic Dis 1982). All analyses were repeated for the age subgroups of < 65 vs ≥ 65 years.
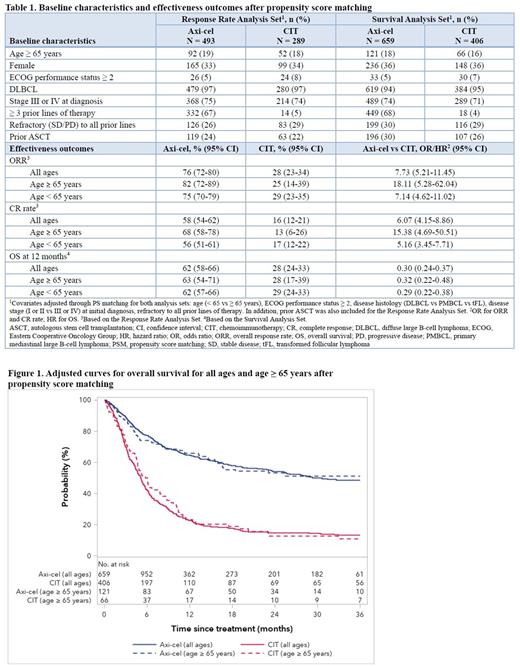
Results: Overall, pts who received axi-cel were older (median age 62.3 vs 55.4 years for CIT), more likely to have an ECOG performance score of ≤ 1 prior to treatment (96% vs 85% for CIT), receive more lines of prior treatment (66% with ≥ 3 prior lines of therapy vs 4% for CIT) or remain refractory to all prior lines of therapy (33% vs 28% for CIT). The imbalance in observed baseline characteristics was reduced after PS matching (Table 1).
At a median follow-up of 24 months for the axi-cel group and 60 months for the CIT group, ORR was 76% (CR 58%) for pts who received axi-cel vs 28% (CR 16%) for those who received CIT in the PS-matched sets. At 12 months, OS was 62% for axi-cel recipients vs 28% for pts receiving CIT. A 57% difference in ORR (55% difference in CR) favoring axi-cel was observed among pts aged ≥ 65 years vs 46% difference in ORR (39% difference in CR) in pts aged < 65 years (Table 1).
Based on multivariable analyses in the PS-matched sets, axi-cel was associated with significantly increased ORR and CR compared with CIT (odds ratio [OR] 7.73 [95% CI 5.21-11.45] for ORR; OR 6.07 [95% CI 4.15-8.86] for CR) after adjusting for pre-specified key prognostic factors. Increased magnitude of benefit in response rates for axi-cel vs CIT was also observed among elderly pts (ORR: OR 18.11 [95% CI 5.28-62.04] for age ≥ 65 years vs 7.14 [95% CI 4.62-11.02] for age < 65 years; CR: OR 15.38 [95% CI 4.69-50.51] for age ≥ 65 years vs 5.16 [95% CI 3.45-7.71] for age < 65 years) (Table 1). Axi-cel was associated with significantly increased OS across all ages (hazard ratio [HR] 0.30 [95% CI 0.24-0.37]) as well as in pts aged ≥ 65 years (HR 0.32 [95% CI 0.22-0.48]). Sensitivity analyses based on conventional multivariable logistic and Cox regression in the unmatched population showed similar results.
Conclusion: This is the first study to estimate the real-world effectiveness of axi-cel compared with CIT in pts with r/r LBCL after ≥ 2 lines of therapy. Axi-cel provides clinically meaningful benefits to pts of all ages in real-world settings, notably with a larger relative benefit in response rates of axi-cel over CIT in older pts. These findings further support the broader use of axi-cel in the elderly population.
MAL and H-LW contributed equally.
Disclosures
Lunning:Morphosys: Consultancy; Astra-Zeneca: Consultancy; Pharmacyclics: Consultancy; Astellas: Consultancy; BMS: Consultancy, Research Funding; TG Therapeutics: Consultancy; ADC Therapeutics: Consultancy; Diiachi-Sankyo: Consultancy; Janssen: Consultancy; Kite, a Gilead Company: Consultancy; CURIS: Research Funding; Seattle Genetics: Consultancy; Genmab: Consultancy; Nurix Therapeutics: Consultancy; Genentech: Consultancy; EUSA: Consultancy; AbbVie: Consultancy; Acrotech: Consultancy; Fate Therapeutics: Consultancy. Wang:Kite, a Gilead Company: Current Employment. Hu:Kite, a Gilead Company: Current Employment. Locke:Society for Immunotherapy of Cancer: Other: Education or editorial activity; BioPharm Communications: Other: Education or editorial activity; CERo Therapeutics: Research Funding; ), National Cancer Institute: Research Funding; Leukemia and Lymphoma Society: Research Funding; Aptitude Health: Other: Education or editorial activity; ASH: Other: Education or editorial activity; Takeda: Consultancy; Sana: Consultancy; Daiichi Sankyo: Consultancy; CAREducation: Other: Education or editorial activity; Clinical Care Options Oncology: Other: Education or editorial activity; Imedex: Other: Education or editorial activity; BMS: Research Funding; A2: Consultancy; Celgene: Consultancy; Other: Patents & Royalties: patents, royalties, other intellectual property from several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy.; Wugen: Consultancy; Umoja: Consultancy; Novartis: Consultancy, Research Funding; Legend Biotech: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Janssen: Consultancy; Iovance: Consultancy; GammaDelta Therapeutics: Consultancy; Emerging Therapy Solutions Gerson Lehrman Group: Consultancy; EcoR1: Consultancy; Cowen: Consultancy; Calibr: Consultancy; Cellular Biomedicine Group: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Bluebird Bio: Consultancy, Research Funding; Allogene: Consultancy, Research Funding; Amgen: Consultancy. Siddiqi:TG Therapeutics: Research Funding; Oncternal: Research Funding; Seattle Genetics: Speakers Bureau; Janssen: Speakers Bureau; PCYC: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Jacobson:BMS/Celgene: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Other: Travel Support; Pfizer: Other: Travel Support, Research Funding; Daiichi Sankyo: Consultancy, Honoraria; Celgene: Other: Travel Support; Humanigen: Consultancy, Honoraria, Other: Travel Support; Axis: Speakers Bureau; Clinical Care Options: Speakers Bureau; AbbVie: Consultancy, Honoraria; Precision BioSciences: Consultancy, Honoraria, Other: Travel Support; Nkarta: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; ImmPACT Bio: Consultancy, Honoraria; Instil Bio: Consultancy, Honoraria; bluebird bio: Consultancy, Honoraria; Ispen: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Lonza: Consultancy, Honoraria, Other: Travel Support. Ahmed:Servier: Membership on an entity's Board of Directors or advisory committees; Chimagen: Consultancy, Research Funding; Xencor: Research Funding; Seagen: Research Funding; Myeloid Therapeutics: Consultancy; Merck: Research Funding; Tessa Therapeutics: Consultancy, Research Funding. Miklos:Janssen: Consultancy, Honoraria; Kite, a Gilead Company: Research Funding; Adaptive Biotech: Consultancy; Bristol Meyers Squibb: Consultancy; Fosun Kite: Consultancy, Honoraria; Pharmacyclics: Patents & Royalties: cGVHD Ibrutinib patent ; Novartis: Consultancy; Allogene: Research Funding. Lin:Bluebird Bio: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Sorrento: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Legend: Consultancy; Novartis: Consultancy; Juno: Consultancy; Janssen: Consultancy, Research Funding; Gamida Cell: Consultancy; Vineti: Consultancy; Merck: Research Funding; Takeda: Research Funding. Hill:Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding. Ghobadi:Wugen Inc: Consultancy; Celgene: Consultancy; Atara: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Research Funding. Neelapu:Medscape: Consultancy, Honoraria; Precision Biosciences: Consultancy, Honoraria, Other: Personal fees, Research Funding; Aptitude Health: Consultancy, Research Funding; Merck: Consultancy, Honoraria, Other: Personal fees, Research Funding; Incyte: Consultancy, Honoraria, Other: Personal fees; Allogene Therapeutics: Consultancy, Honoraria, Other: Personal fees, Research Funding; Kite: Consultancy, Honoraria, Other: Personal fees, Research Funding; Legend Biotech: Consultancy, Honoraria, Other: Personal fees; Bluebird Bio: Consultancy, Honoraria; Cell Medica/Kuur: Consultancy, Honoraria, Other: Personal fees; Calibr: Consultancy, Honoraria, Other: Personal fees; Unum Therapeutics: Consultancy, Honoraria, Other: Personal fees, Research Funding; Adicet Bio: Consultancy, Honoraria, Other: Personal fees, Research Funding; Pfizer: Consultancy, Honoraria, Other: Personal fees; Celgene: Consultancy, Honoraria, Other: Personal fees, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Other: Personal fees, Research Funding; Novartis: Consultancy, Honoraria, Other: Personal fees; Bio Ascend: Consultancy, Honoraria; Poseida: Research Funding; Cellectis: Research Funding; Karus Therapeutics: Research Funding; Acerta: Research Funding; Takeda Pharmaceuticals: Patents & Royalties: related to cell therapy.. Westin:Kite, a Gilead Company: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; MorphoSys/Incyte Corporation: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; Merck: Consultancy; Iksuda: Consultancy; Calithera: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; MonteRosa: Consultancy; AstraZeneca: Consultancy, Research Funding; Abbvie/GenMab: Consultancy; SeaGen: Consultancy. Miao:Gilead Sciences: Current holder of stock options in a privately-held company; Kite, a Gilead Company: Current Employment. Shahani:Kite, a Gilead Company: Current Employment, Current holder of stock options in a privately-held company; Amgen: Speakers Bureau. Patel:Kite, a Gilead Company: Current Employment; Gilead: Current holder of stock options in a privately-held company. Spooner:Gilead Sciences: Current holder of stock options in a privately-held company; Kite, a Gilead Company: Current Employment; Delta Hat Limited: Other: Support with conception, analysis and manuscript writing. Commissioned by Kite, a Gilead company. Fu:Kite, A Gilead Company: Current Employment, Current holder of stock options in a privately-held company; Amgen: Current holder of stock options in a privately-held company; Cellares: Patents & Royalties: Intellectual property. Xu:Kite, a Gilead Company: Current Employment. Pasquini:Kite: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Janssen: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal